How Many Electrons Are Gained in the Half Reaction O2
How many electrons are gained in the half-reaction O2 electrons 2O2. What is a balanced equation for this redox reaction.
The reaction can be regarded as a decomposition of PbO2 PbO2 - PbO 12 O2 coupled with an acid-base reaction PbO 2 HBr - PbBr2 H2O so that the balanced reaction is PbO2 2HBr - PbBr2 12 O2 H2O.

. How many electrons are gained or lost in the following half-reaction. Chemistry 03052021 1640 neariah24. How many electrons are gained or lost in the following half-reaction.
Write out half reactions to show how many electrons are gained or lost by each species. 12 electron is gained. In the half reaction where an oxygen molecule becomes into two oxide ions it obtains 4 electrons from the oxidising substance.
Oxygen gains 3 electrons e. 2 electrons are gained. 12 electron is lost.
Combustion involves the transfer of electrons to oxygen which has an extremely high tendency for gaining electrons. In the reaction how many electrons does an atom of oxygen lose or gain. Hence the balanced equation for the reaction is.
The number of electrons shuffled in the reaction is not chosen arbitrarily but is based on the initial and final oxidation numbers of the elements in the reaction after the equations are balanced. 12 electron is lost. How many electrons in total are transferred from iron atoms to oxygen atoms in the formation of two units of iron III hydroxide Fe OH3.
Oxygen gains 2 electrons c. Using this guideline the oxidation half. There is no loss or gain of electrons _D___ 8.
The reduction half-reaction requires 6 e- while the oxidation half-reaction produces 2 e-. The sixth step involves multiplying each half-reaction by the smallest whole number that is required to equalize the number of electrons gained by reduction with the number of electrons produced by oxidation. In other words 2 moles of electrons are transferred for making 1 mole of hydrogen gas.
What are two half reactions to show how many electrons are gained or lost by each species. You need to have 6H to balance out the Hydrogens. So lets check it up.
In the half reaction where an oxygen molecule becomes into two oxide ions it obtains 4 electrons from the oxidising substance. 2 electrons are gained. The half-cell reaction for hydrogen reduction can be written as.
Two moles of magnesium Mg reacted with one mole of to give two moles of. 2 Na. Zn Zn2 2 electrons are lost.
Asked Aug 7 2019 in Environmental Atmospheric Sciences by mmjl70. How many electrons are gained in the half-reaction O2 electrons 202. Step 1 of 3.
2 electrons are lost. 2 electrons are lost. For the second half reaction you need to have 3 waters to balance out the oxygens.
That is 2 electrons are transferred for making 1 molecules of hydrogen gas from protons. Then so we need to add 6 electrons to the product side of this second half-reaction to make it so that they are equal charges. 12 electron is lost.
In this process the metal Mg is oxidized to thus lose of two electrons from each Mg two oxygen atoms gain a total of four electrons are reduced to 2. How many electrons are gained in the half-reaction O2 electrons 20 Ο Α. Zns 2 Haq Zn2aq H2g Oxidation Reaction.
Solution for How many electrons have been gained by vanadium in the given half reaction. O2 2Zn --2ZnO. How many electrons are gained or lost in the following half-reaction.
Of course the decomposition reaction is redox while the. Cl2 2 Cl-. How many electrons are gained in the half-reaction O2 electrons 2O2.
The reaction of aluminum metal Al with oxygen O2 forms Al2O3. Click hereto get an answer to your question How many electrons are involved in the following redox reaction. Oxygen loses 3 electrons d.
What is a balanced equation for this redox reaction. Hence we have 4Fe3 and 2O32- showing twelve electrons lostgained. Write out half reactions to show how many electrons are gained or lost by each species.
Separate this redox reaction into its component half-reactions. So that means that the first half-reaction we need to multiply. The only stable compound with formula ceSnSO_4 is made of ceSn2 and ceSO_42- ions.
V5 aq Mg s V s Mg2 aq Answer. Cr2O72 - Fe2 C2O42 - Cr3 Fe3 CO2 Unbalanced. Answer 1 of 6.
What are two half reactions to show how many electrons are gained or lost by each species. There are 10 electrons transferred in the following reaction. Correct answer - How many electrons are gained in the half-reaction 02 electrons 202-.
Group of answer choices. 12 electron is gained. Then you double check the charges.
12 electron is gained. The reaction of aluminum metal Al with oxygen O2 forms Al2O3. If we look at the product side we will notice that iron lost twelve electrons and oxygen gained twelve electrons.
Oxygen loses 2 electrons b. VO3 V2- O 5 O 1 3. Identify the species that is oxidized and the species that is reduced in the reaction.
4 electrons are gained.

Oxidation And Reduction In Terms Of Electron Transfer Redox Equilibrium Youtube

Identify The Species That Is Oxidized And The Species That Is Reduced In The Following Reaction Write Out Half Reactions To Show How Many Electrons Are Gained Or Lost By Each Species

Chapter 19 Redox Reactions And Electrochemistry Notes Answers
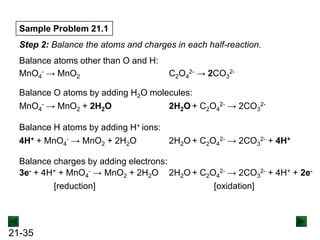
New Chm 152 Unit 7 Power Points Su13
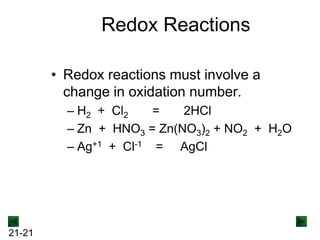
New Chm 152 Unit 7 Power Points Su13

What Is Redox Oxidation Chemistry Reduction

Writing Redox And Half Reactions

4 37f Balance The Half Reaction So32 Aq So42 Aq In Acidic Solution Youtube
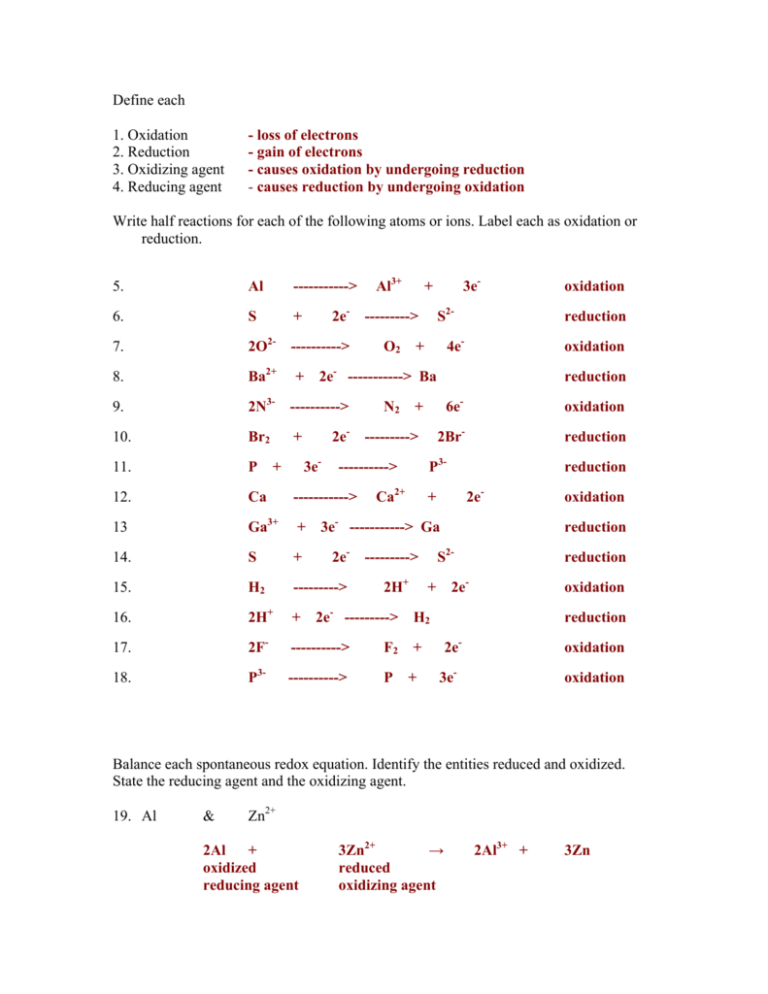
Define Each 1 Oxidation Loss Of Electrons 2 Reduction

Chemistry Reduction And Oxidation Reactions Wikiversity
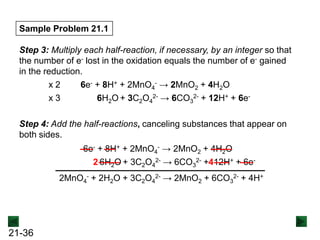
New Chm 152 Unit 7 Power Points Su13

New Chm 152 Unit 7 Power Points Su13
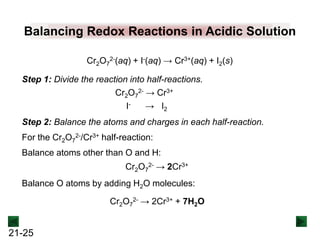
New Chm 152 Unit 7 Power Points Su13
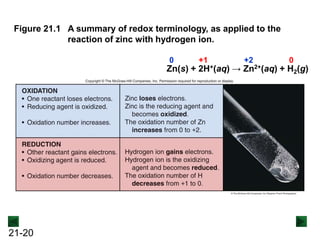
New Chm 152 Unit 7 Power Points Su13

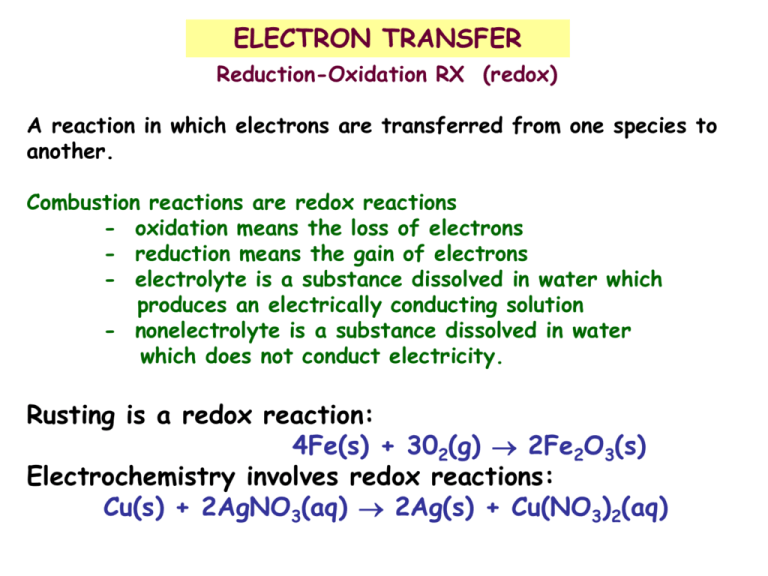



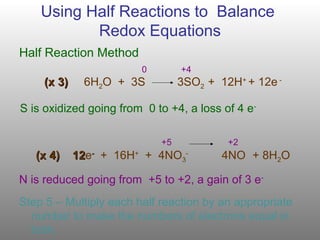
Comments
Post a Comment